Deaths caused by malaria this year
Grant Agreement number: RIA2018SV-2311 PfTBV RIA2018SV


PRESENTATION
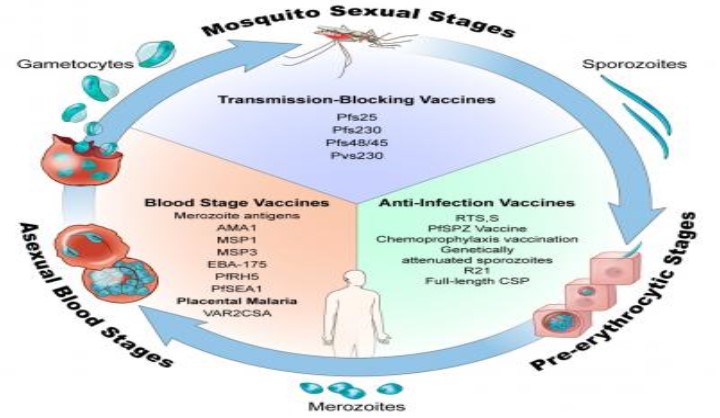
The PfTBV Malaria Blocking Transmission Consortium was formed in 2019 to develop and execute on a strategy to compare, select and develop malaria vaccine candidates. Using innovative trial designs, the consortium will 1) accelerate well-established candidates into the clinic for direct comparison and evaluation 2) select the most efficacious malaria vaccine to stop transmission of Plasmodium falciparum (Pf) from humans to mosquitoes, and 3) establish assays, endpoints, and regulatory pathways for definitive efficacy assessment of a transmission blocking vaccine (TBV).
“This project (RIA2018SV-2311) is part of the EDCTP2 programme supported by the European Union”.
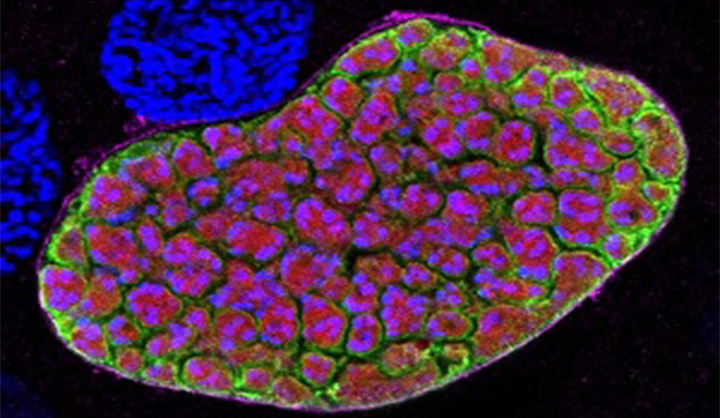
Malaria
The burden of malaria remains intolerable, causing over 200 million clinical cases and 400,000 deaths each year, with pregnant women and children in Africa bearing the greatest risk. Malaria is caused by parasites transmitted by mosquitoes. It can cause high fever, chills, flu-like symptoms, and severe anemia and can infect your liver and blood.READ MORE
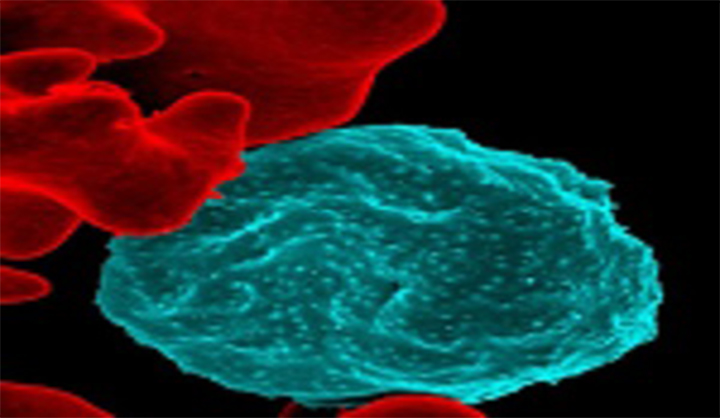
Malaria Vaccines
Vaccines are powerful tools that could accelerate malaria elimination efforts. Transmission-blocking vaccines (TBVs) offer a new approach by targeting developing parasites in the mosquito host (a bottleneck in the malaria parasite lifecycle) and thereby contributing to malaria elimination and potentially eradication.READ MORE
WELCOME NOTE OF THE PFTBV COORDINATOR
Dear colleagues and partners, Ladies and Gentlemen, Dear readers,
In my role of Coordinator, I am pleased to launch the website dedicated to «PfTBV Project".

This platform offers an opportunity for regular exchange on the implementation status of this challenging but exciting project and helps to share experiences and best practices as well as innovative ideas for our future development.
On behalf of my colleagues in the PfTBV consortium, I would like to sincerely thank the EDCTP for granting us a direct grant of Eighteen Millions Euro(18€) for this project, but also to the United States of America (NIH and PATH) for their indirect contribution of Fourteen million two hundred nineteen four thousand euros (14,294,000€ ).
This project aims to develop vaccines that can block the transmission of malaria by cutting the transmission chain. In other words, when a person is vaccinated, the vaccine will then prevent mosquitoes from transmitting malaria to other people.
PfTBV Project Coordinator
Three promising new malaria vaccines are being evaluated in collaboration with researchers from Burkina Faso, Guinea, Liberia and Mali, including researchers from 2 European countries (Denmark, Holland) and three institutions from the United States of America, (the National Institutes of Health, NIH and PATH).I would like to reiterate our thanks to our partners for their financial and technical supports as well as their commitment for the success of this common endeavour – i.e.combating malaria in general and in kids in particular.
If successful, these promising candidate vaccines will definitely revolutionize the fight against malaria, which death toll is still heavy in endemic countries. We warmly welcome and appreciate this North-South cooperation and we are fully determined to carry out the various studies included in this project. We are truly thankful to our respective authorities for their support to this project.
I am convinced and I hope that working closely together, we will add our value to the management of malaria in Africa.
I look forward to seeing your posts, news and suggestions for the improvement of this website.
PfTBV Project Coordinator
Professor Issaka Sagara
BUILDING CAPACITY FOR THE COMPARATIVE EVALUATION OF MALARIA VACCINE CANDIDATES IN SUB-SAHARAN AFRICA

Capacity development and technology transfer that accelerates malaria vaccine development:
- Consortium brings together key opinion leaders in malaria vaccine trial conduct and immunology to build capacity for state-of-the-art vaccine trials
- Interdisciplinary networking will enable sharing of best practices across four clinical trial sites, strengthening the ability of each site to conduct these trials and future research.
- Site harmonization, establishment of procedures according to GCP, and capacity for data generation, storage and modeling can be employed for other vaccine targets beyond malaria

- Foster a regional approach to collaborative trials among national research and regulatory bodies.
EVENTS
Financial partners
Coordinator